- Αρχική
- Η Εταιρεία
- Υπηρεσίες
- Κατ' Οίκον Ανάλυση
- Chem-Zine
- Πελάτης
- ΠΕΙΤΕ ΜΑΣ ΤΗ ΓΝΩΜΗ ΣΑΣ
- Blog
Nutrient competition in fertilizers
Δείτε επίσης:
In the soil and on the leaves there is serious competition between nutrients and this can create serious problems in their availability. In short, there are serious competition phenomena and this is why we often end up with incorrect fertilizations with a direct consequence of wasting money and the irrational use of fertilizers. There are two important phenomena that are created: one of competition in which a high content of one nutrient creates problems in the absorption of another nutrient and the phenomenon of stimulation where high levels of one nutrient create a need for additional reinforcement of another nutrient.
For this reason, it is very important to calculate the nutrient ratios in the soil (especially magnesium to potassium, calcium to magnesium and calcium to magnesium and potassium) and the total nutrient ratios in the leaves (nitrogen to total nutrient, potassium to total nutrient and phosphorus to total nutrient).
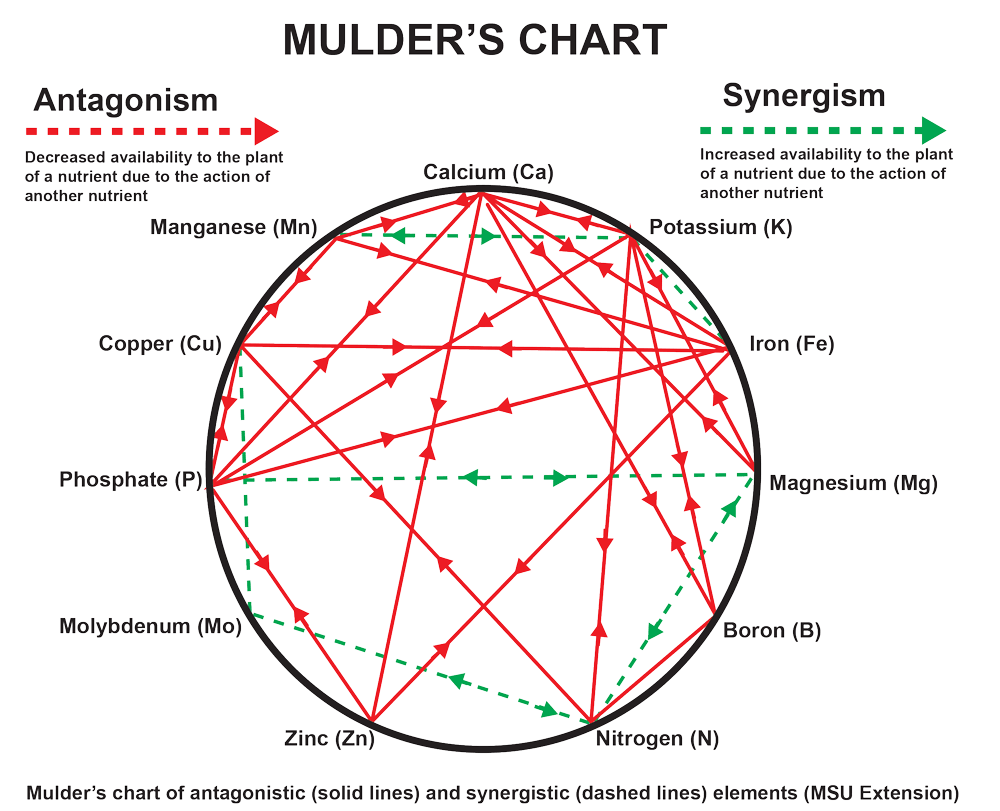
All of this is summarized in the Mulder diagram and in the international bibliography. Let's look at the most well-known interference problems:
1. Calcium competes with magnesium, potassium, phosphorus, iron, boron and zinc
2. Magnesium competes strongly with calcium and potassium.
3. Potassium competes strongly with magnesium, while in the foliage we have strong competition with phosphorus and nitrogen.
4. Iron competes very strongly with calcium.
5. Boron acts strongly competitively with nitrogen.
6. Phosphorus competes strongly with calcium, while in the foliage it is inhibited by an excess of nitrogen and potassium.
These specific obstacles are directly dependent on the form of the formulations and the soil pH. For example, in the case of urea (an organic form of nitrogen that is directly converted to ammonia), boron helps to bind the ammonia so that it does not evaporate immediately. However, nitrogen is not taken up from ammonia and boron at the same time, but the majority of boron is not absorbed.
If you like our articles, follow us on facebook and subscribe to our youtube channel
For more information please fill out the Contact Form below or call us at 2231 2231 99 from 9:00 to 17:00.
Ενημερωτικά Δελτία

Η Εταιρεία
Το Γενικό Χημείο Έρευνας και Αναλύσεων Πασιάς – Ραπτοπούλου είναι ένα σύγχρονο Κέντρο που δραστηριοποιείται στον κλάδο των χημικών αναλύσεων και της έρευνας.
Τυμφρηστού 181 Λαμία
+30.2231.2231.99
+30.694.954.3858











